This year another young researcher has joined the Doctor of Sciences list at the Institute of High Temperature Electrochemistry. On May 22, 2019 Dmitry A. Medvedev has successfully defended his doctoral thesis according to the speciality 02.00.05 – electrochemistry. The subject of the dissertation was the study of high temperature proton electrolytes based on Ba (Ce, Zr) O3 with the perovskite structure: synthesis strategies, optimization of properties and features of their application. Dmitry has received confirmation from the VAK Commission related to the issuance of a diploma of Doctor of Chemical Sciences already on October 14.
Dmitry Medvedev spoke about how the defense and preparation for it went, and shared some future plans with us.
– My doctoral dissertation, as well as my Ph.D. thesis, is devoted to obtainment of novel proton-conducting materials and to the study of their properties. These are unusual objects in the oxide system family. The uniqueness lies in the fact that their pronounced proton transport is due to the presence of proton charge carriers (defects), which are not elements of the basic structure, but appear in it under certain conditions.
This feature allows using the proton-conducting oxides in electrochemical devices for various purposes: for converting the chemical energy of hydrogen-containing fuels into electricity (solid oxide fuel cells, SOFC), for reverse conversion (solid oxide electrolyzers, SOC), for determining the concentration of hydrogen or water vapor in gas mixtures at high temperatures (sensors), for the separation of hydrogen (pumps) with the purpose of its further compression (compressors), etc.
Among many proton-conducting materials I chose one of the most attractive (barium cerate, barium zirconate and solid solutions based on them), and then conducted research focused on the optimization of their functional properties for the use in SOFC.
At the beginning of my scientific career (2005–2007), we drew attention to the problem of obtaining gas-tight ceramics based on BaCeO3. Our research team was one of the first to propose a method of introducing small amounts of special additives in order to improve the materials sintering. At that time only three scientific groups (Matsumoto, Tretyakova and Haile) conducted similar studies, which allowed us to be on the crest of the wave along with them. I prepared and defended my dissertation in 2012 based on these results.
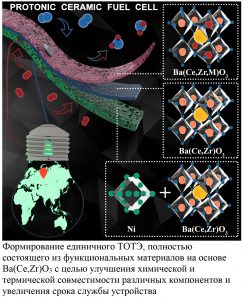
The next step was to achieve a compromise between chemical stability, thermal and electrical properties of proton-conducting oxides. For this purpose we carried out a partial replacement of cerium with zirconium in BaCeO3. On the one hand, this made it possible to suppress phase transitions characteristic of cerate, stabilizing the cubic structure of the modified compounds already at room temperature, and also increasing their chemical stability with respect to undesirable processes of the formation of barium hydroxide and carbonate.
On the other hand, we tried to maintain a sufficient level of ionic conductivity of the obtained compounds, since zirconates, due to the higher density of grain boundaries, have low resulting conductivity than cerates.
The final task during preparing the doctoral thesis for defense was the demonstration of the possibility of the developed electrolytes application in electrochemical devices. For this, at the first stage we adapted the calendering method in order to obtain semi-elements based on thin-layer proton-conducting electrolytes at medium high annealing temperatures, 1400–1450 ° С (studies are also can be found in the PhD thesis of Yu.G. Lyagaeva). The success of this direction was inextricably connected with previous results, that is with the possibility of forming dense layers of Ba (Ce, Zr) O3 by adding a sintering additive as well as by achieving thermochemical compatibility of functional materials due to varying the electrolyte composition and the rational selection of electrode systems.
Along with scientific work, we also carried out basic research related to optimizing the SOFC operation by varying the temperature and partial pressure of water vapor in the cathode and anode spaces (which mainly determine proton transport in oxides). These results were not included in my doctoral thesis, but will form the basis of the PhD thesis of the graduate students N.A. Danilova, A.P. Tarutin and L.R. Tarutina.
It is worth noting that over the past ten years the study of proton-conducting oxides has become the mainstream not only in Russia, but also globally in the field of high temperature electrochemistry. This is evidenced by a list of new reviews reflecting the latest achievements in this field:
Fagg et al. https://doi.org/10.1016/j.jpowsour.2019.226991
Brinkman et al. https://doi.org/10.1007/s10853-019-03559-9
Kim et al. https://doi.org/10.1016/j.rser.2019.04.042
Somalu et al. https://doi.org/10.1016/j.ceramint.2019.01.045
Bi et al. https://doi.org/10.1016/j.elecom.2018.09.001
Sunarso et al. https://doi.org/10.1016/j.ijhydene.2018.06.045
Chen et al. https://doi.org/10.1002/adfm.201903805
We keep abreast on them:
https://doi.org/10.1002/adfm.201802592
https://doi.org/10.1016/j.ijhydene.2019.08.130
Looking back, I joke that I could defend another doctoral thesis on the basis of the available data, since the technical process of the formation of work, as well as the collection and preparation of the relevant documents, were in relaxation mode for me, without any excesses, due to the speed of perception and processing of information. However, there were some difficulties. The first is psychological: you just need to force yourself to start writing a dissertation. The excuses “later” and “next week” do not work.
This problem, of course, is not new, but mentioning it once again will not be useless. The second difficulty was the formation of the integrity of the work, the silhouette of which began to appear only when more than half was written. It was not easy to find this “common thread”, since the synthesis of your published results into a single whole is as complicated as the synthesis of a complex oxide from two simple ones: if you don’t know the peculiarities of the starting materials and the reaction conditions, nothing will work.
2008–2019 can be called a life-changing in my scientific career. During this time I became an expert of the Russian Science Foundation, was invited as a permanent editor in the International Journal of Hydrogen Energy, and several times acted as a guest editor for such well-known magazines as Energies, Materials, Journal of Solid State Electrochemistry and Electrochimica Acta. I am not a fan of attending conferences frequently, and therefore communication in the scientific community develops through the directions noted above and through correspondence with colleagues.
I think this is quite effective, because the citation of our work has increased (especially in the last few years), partly owing to international research conducted with professors P. Tsiakaras (Greece), J. Shao (USA) and A. Yaremchenko (Portugal).
In conversations with colleagues sometimes the idea flickers that everything has already been studied in the field of proton-conducting materials. But I know that there are many questions that do not have clear answer. In the group of the head of our laboratory A.K. Demin we see them and try to solve systematically.
For example, now we have focused on electrode processes that occur in systems with proton-conducting electrolytes. we are designing new electrolyte-electrode pairs in order to understand them, trying to minimize the number of negative factors affecting the characteristics of the electrodes. From the point of view of materials science, we synthesize new oxide electrodes (without cobalt and / or alkaline earth elements) and test their suitability in the mentioned systems (see figure →).
In collaboration with colleagues from other laboratories of our institute (V.I. Tsidilkovsky, L.P. Putilov, A.S. Farlenkov), we continue the synthesis of new electrolytes and a more detailed study of their conductivity nature.
As for me, this topic is interesting, although the work on it is not sufficiently cited due to the relatively narrow circle of readers and experts. Hence, it is difficult to grow in terms of scientometric indicators in this area. But we are trying.
Below are the most important articles published in 2019.
-
N. Danilov, J. Lyagaeva, G. Vdovin, D. Medvedev. Multifactor performance analysis of reversible solid oxide cells based on proton-conducting electrolytes. Applied Energy. 2019. V. 237. P. 924–934. https://doi.org/10.1016/j.apenergy.2019.01.054.
-
A. Tarutin, J. Lyagaeva, A. Farlenkov, S. Plaksin, G. Vdovin, A. Demin, D. Medvedev. A reversible protonic ceramic cell with symmetrically designed Pr2NiO4+δ-based electrodes: fabrication and electrochemical features. Materials. 2019. V. 12. № 1. No. 118. https://doi.org/10.3390/ma12010118.
-
E. Pikalova, A. Kolchugin, M. Koroleva, G. Vdovin, A. Farlenkov, D. Medvedev. Functionality of an oxygen Ca3Co4O9+δ electrode for reversible solid oxide electrochemical cells based on proton-conducting electrolytes. Journal of Power Sources 2019. V. 438. No. 226996. https://doi.org/10.1016/j.jpowsour.2019.226996.
-
D. Medvedev. Trends in research and development of protonic ceramic electrolysis cells. International Journal of Hydrogen Energy. 2019. V. 44. № 49. P. 26711–26740. https://doi.org/10.1016/j.ijhydene.2019.08.130.
-
A. Tarutin, A. Kasyanova, J. Lyagaeva, G. Vdovin, D. Medvedev. Towards high-performance tubular-type protonic ceramic electrolysis cells with all-Ni-based functional electrodes. Journal of Energy Chemistry. 2020. V. 40. P. 65–74. https://doi.org/10.1016/j.jechem.2019.02.014.